EO-Flexible Chamber Technology Sets Us Apart
Ethylene Oxide-Flexible Chamber Technology® is Andersen’s proprietary and award-winning sterilization process. It is shared by the Andersen Anprolene® and EOGas 4® models. EO-FCT allows our sterilizers to use almost 90% less gas per cycle than any other system in the world.
(The process for the EOGas 3 is slightly different.)

1. Load Preparation
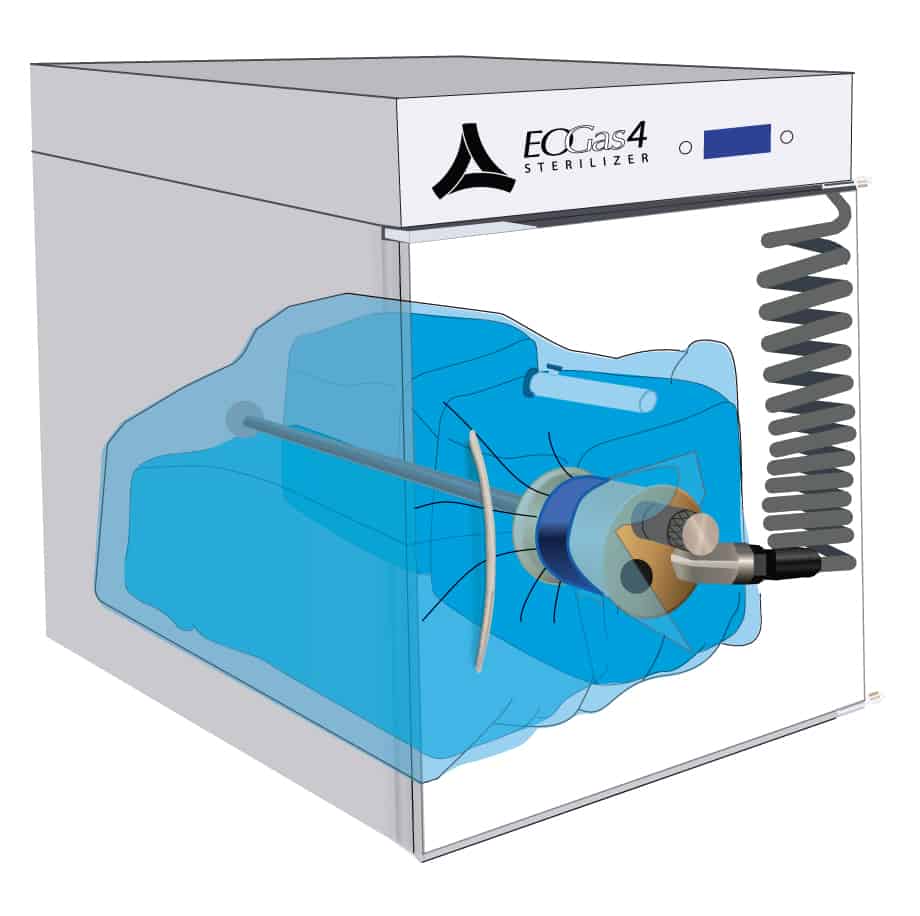
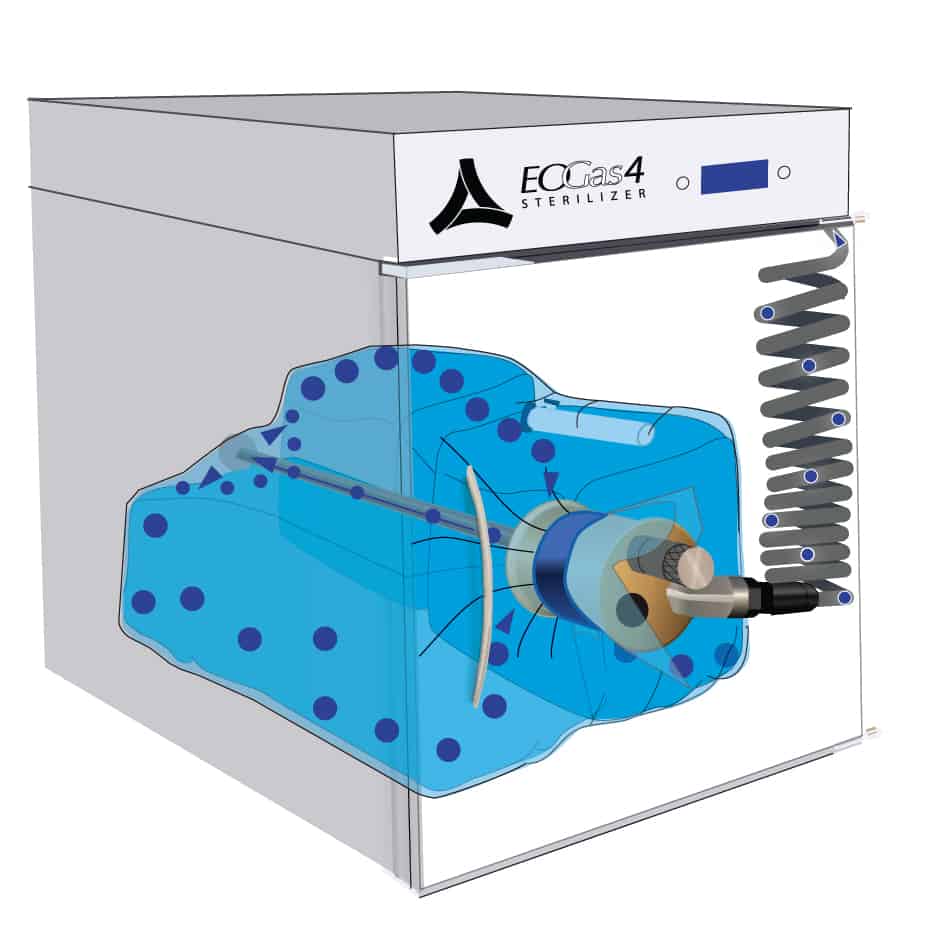
This cut-away of an Andersen sterilizer reveals a loaded sterilization bag — the “Flexible Chamber” — secured with a blue strap around the purge probe.
2. Air Purge
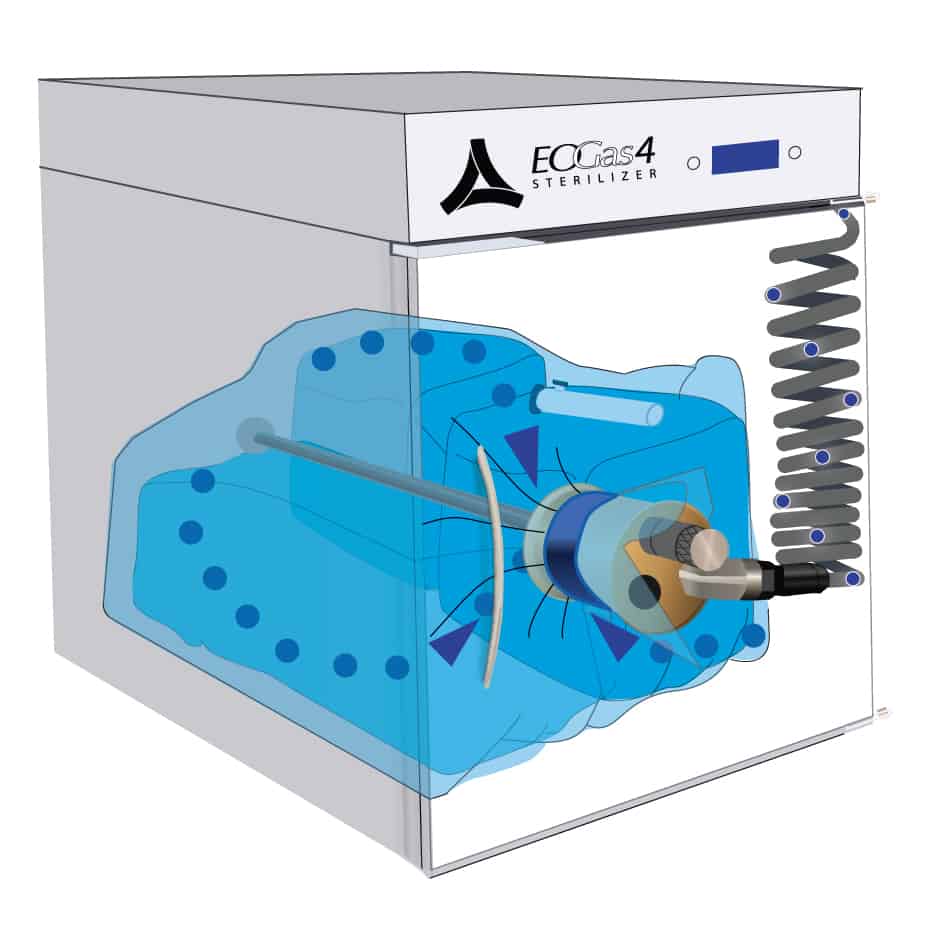
The sterilization cycle begins by purging excess air from the flexible chamber.
Air molecules, represented by the blue dots, are exhausted from the sterilization bag through the purge probe and purge tube.
With excess air removed, the ethylene oxide (EO or EtO) concentration in the sterilization bag is maximized during sterilization, thereby minimizing the amount of EO work needed for effective sterilization.
3. Cartridge Activation & Sterilization
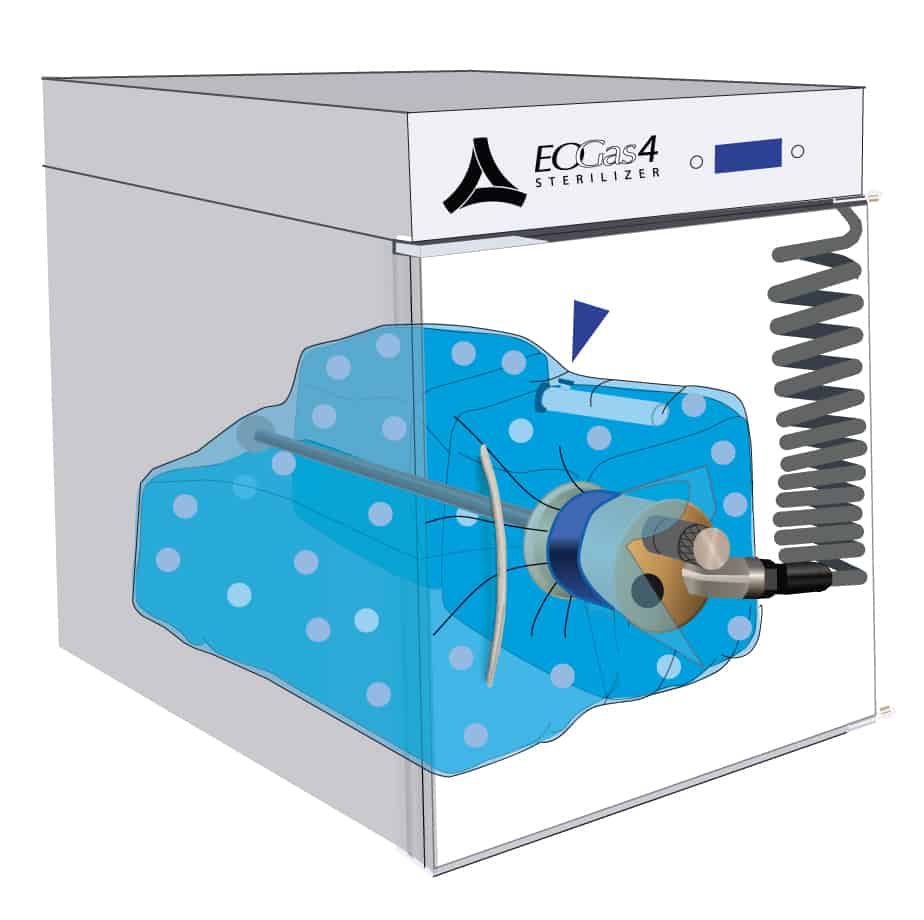
Once the initial purge is complete, the gas cartridge is manually activated by pressing the button on the cartridge through the wall of the sterilization bag.
Ethylene oxide, represented by the purple dots, spreads throughout the sterilization bag and permeates the items being processed. The gas-impermeable flexible chamber maintains a high gas concentration during the sterilization cycle.
4. Gas Ventilation
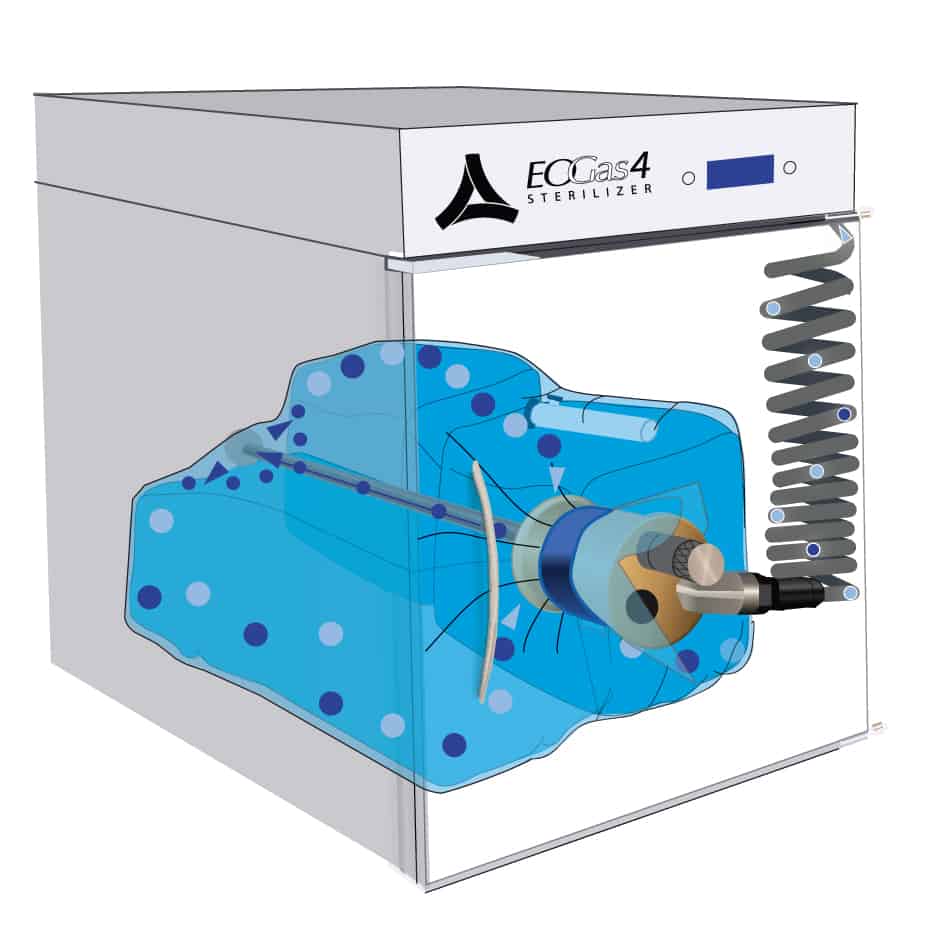
At the end of the sterilization cycle, the process of ventilating the sterilization bag begins. A valve in the purge probe opens, allowing fresh air, represented by blue dots, to flow down the probe and into the back of the bag. Concurrently, ethylene oxide, represented by purple dots, is exhausted through the same probe.
This effective cycle demonstrates how EO works to ensure the safe and thorough sterilization of medical equipment. By removing all residual gas, the system safeguards the environment and the users, showcasing the efficiency and safety of EO sterilization technology.
5. Active Aeration
Conclusion
Aeration continues after ventilation, flushing air through the sterilization bag and out the purge tube. Aeration of the devices in the sterilization bag ends when the operator exits the cycle to remove the sterilization bag from the sterilizer.
Andersen’s next-generation EO sterilization process has outclassed all others.
EO-FCT unique benefits include:
- Low temperature
- No steam injection
- No deep vacuum (which can damage delicate medical instruments)
- Ultra-efficient use of sterilant
- Less gas per cycle
These improvements create a zero-damage terminal sterilization process that has been exhaustively tested and FDA-cleared.
Please contact us to learn more.
