The Food and Drug Administration (FDA) announced it is investigating reports of infections associated with reprocessed urological endoscopes.
From January 1, 2017 to February 20, 2021 the Food and Drug Administration (FDA) received more than 450 medical device reports (MDRs) describing post-procedure patient infections or other contamination issues associated with reprocessing urological endoscopes, including cystoscopes and uretoeroscopes—devices used to view and access the urinary tract.
“The FDA is investigating potential causes and contributing factors associated with reported infections and contamination issues from reprocessed urological endoscopes. We are very concerned about the three reported deaths—outside of the United States—associated with these infections, and we’re acting fast to communicate with health care providers and the public about what we know and what is still an emerging issue,” said Jeff Shuren, M.D., J.D., director of FDA’s Center for Devices and Radiological Health.
“While some reports indicate the potential causes could be inadequate reprocessing or device maintenance issues, we’re also evaluating other possibilities, including device design or the reprocessing instructions in the labeling. Although we believe that the risk of infection is low based on available data, we’re reminding health care providers how important it is to follow the labeling and reprocessing instructions to properly clean and reprocess the devices, including accessory components. We take all reports of adverse events seriously, and we encourage prompt reporting to the FDA to help us identify and better understand the risks associated with reprocessed medical devices.”
Cystoscopes and ureteroscopes are urological endoscopes that allow health care providers to see and access the urinary tract (e.g., urethra, bladder, ureters and kidneys) during diagnostic and therapeutic procedures.
Full FDA news release.

EOGas 4 FDA 510(k) Resources
Printable Press Release
Newsbrief story
About EOGas 4
EOGas 4 510(k) original clearance
(including urological scopes)
EOGas 4 510(k) new clearance
(longer, smaller diameter scopes – duodenoscopes and colonoscopes)
FDA recognizes gas sterilization of duodenoscopes provides a greater margin of safety than high-level disinfection
The FDA’s announcement is the latest in the agency’s continued work on reprocessing measures ensuring the complete inactivation of highly resistant microorganisms from critical endoscopes and duodenoscopes.
Beginning In 2015, outbreaks of carbapenem-resistant Enterobacteriaceae (CRE), drug-resistant “superbugs,” sickened over 100 patients in medical facilities, resulting in dozens of deaths. CRE was transmitted between patients via contaminated duodenoscopes, despite in some cases proper endoscope cleaning and disinfection in accordance with manufacturers’ instructions.
In response to these outbreaks, FDA recommended that hospitals consider at least one of four supplemental measures, including ethylene oxide sterilization to enhance the safety of duodenoscopes and prevent superbug outbreaks.
The EOGas 4 Presents FDA-cleared terminal Sterilization Option for Scopes Under Investigation, as well as Significantly Longer and Smaller Diameter Scopes
“FDA’s subsequent review of field performance of the four supplemental measures confirmed that EO sterilization was the most effective of the supplemental reprocessing measures, and validation studies showed that it was the only measure that assures the complete inactivation of highly resistant microorganisms.”
FDA
The EOGas 4 was originally cleared in 2015 for sterilization of endoscopes < 1100 mm in working length (bronchoscopes, bronchovideoscopes, cystoscopes, ureteroscopes, choledocoscopes and gastroscopes) and a wide range of other materials.
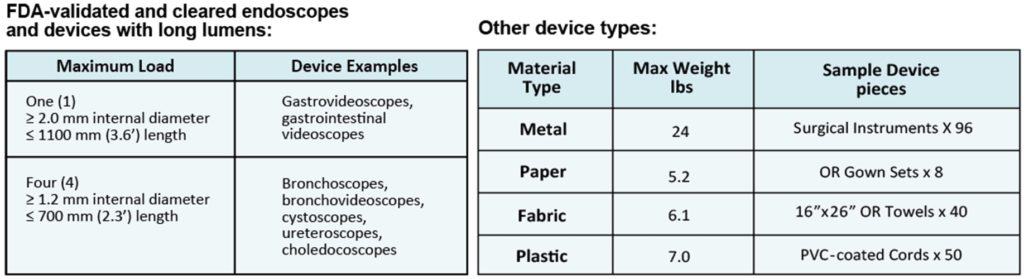
In late 2020, the FDA awarded Andersen Sterilizers’ EOGas 4 series model the first sterilization system 510(k) clearance for terminal sterilization of duodenoscopes and colonoscopes. The new clearance is for endoscopes with > 1100 mm working length (duodenoscopes and colonoscopes) with a maximum lumen length of 3530 mm and minimum lumen diameter of 1.2 mm (that’s up to 11.6 feet long and down to the thickness of a Blu-Ray or CD – less than the height of a dime). Additional clearances were received on chemical indicators, biological indicators and packaging that maintains sterility of endoscopes for up to 6 months after reprocessing.
Devices FDA-Cleared for terminal sterilization in the EOGas 4:
- Gastrovideoscopes, Gastrointestinal videoscopes and similar devices
- Bronchoscopes, bronchovideoscopes, cystoscopes, ureteroscopes, choledocoscopes and similar devices
- Duodenoscopes and colonoscopes: Olympus TJF-Q180V, Olympus TJF-Q160VF, Olympus TJF Q190V, Olympus PJF-160, Fujifilm ED-530XT, Pentax ED34-i10T2, Pentax ED-3490TK, Olympus CF-Q180AL, Fujifilm EC-600HL, Pentax EC-3490Li
Contact our gas sterilization experts today to learn more about Andersen’s FDA 510(k) cleared EOGas 4.
Expert Guidance for Your Needs
Tell us about your sterilization requirements — our experts are here to guide you to the perfect EO solution.