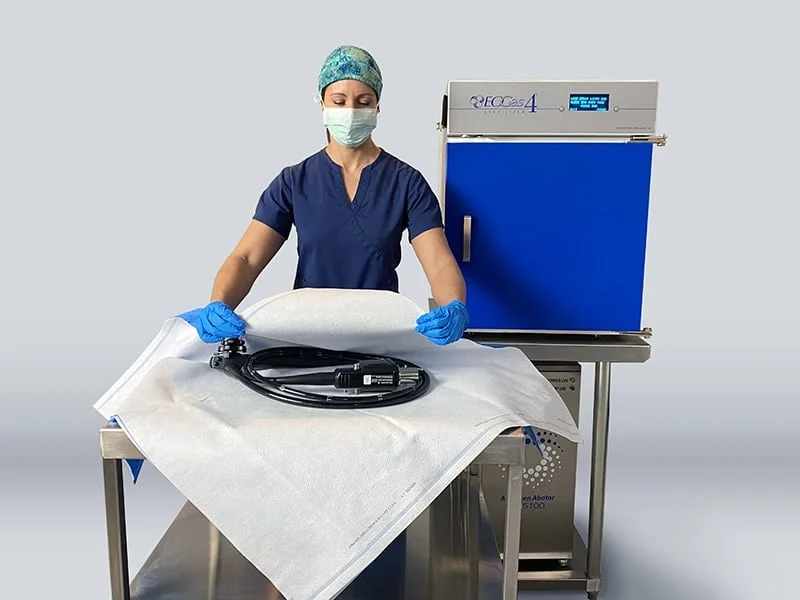
ONLY Sterilization System cleared by FDA 510(k) for Terminal Sterilization of Duodenoscopes & Colonoscopes
Haw River, NC—Andersen Sterilizers, international manufacturer of high-efficiency, flexible chamber, low temperature gas sterilizers, today announced 510(k) clearance by the U.S. Food and Drug Administration (FDA) of its EOGas 4 Ethylene Oxide Gas Sterilizer and associated accessories for sterilizing duodenoscopes and colonoscopes.
The EOGas 4 is labeled to sterilize reusable medical devices that are sensitive to moisture, heat, corrosion or radiation. The EOGas 4 was originally cleared in 2015 for surface sterilization of a wide range of instruments and for sterilization of endoscopes < 1100 mm in working length (bronchoscopes, bronchovideoscopes, cystoscopes, ureteroscopes, choledocoscopes and gastroscopes). The new clearance is for endoscopes with > 1100 mm working length (duodenoscopes and colonoscopes) with a maximum lumen length of 3530 mm (11.6 feet) and minimum lumen diameter of 1.2 mm.
“Prior to clearance of the EOGas 4 system in 2015, we had already started working closely with FDA on the requirements for duodenoscope and colonoscope sterilization,” said Andersen Sterilizers President, William K. Andersen, MD. “This clearance reflects the FDA’s latest validation expectations for terminal sterilization of lumened devices, including those with elevator mechanisms. We also received clearances on chemical indicators, biological indicators, and packaging that maintains sterility of endoscopes for up to 6 months after processing.”
Andersen’s new version of the EOGas 4, featuring a 6-hour cycle, has been put into production immediately upon receiving the clearance.
Sign up below to be the first to know when the new model is available for sale.
EOGas 4 Ethylene Oxide Sterilizer cycle parameters:

The EOGas 4 process sterilizes duodenoscopes and colonoscopes in wrapping materials suitable for EO sterilization (Tyvek®/plastic pouches or Sterisheet® Sterilization Wraps), providing six-months of sterile shelf life for endoscopes after sterilization.
Beginning In 2015, outbreaks of carbapenem-resistant Enterobacteriaceae (CRE), drug-resistant “superbugs,” sickened over 100 patients in medical facilities, resulting in dozens of deaths. CRE was transmitted between patients via contaminated duodenoscopes, despite in some cases proper endoscope cleaning and disinfection in accordance with manufacturers’ instructions. In response to these outbreaks, FDA recommended that hospitals consider at least one of four supplemental measures including ethylene oxide sterilization to enhance the safety of duodenoscopes and prevent superbug outbreaks. FDA’s validation requirements confirm that EO sterilization is the only supplemental reprocessing measure that assures the complete inactivation of highly resistant microorganisms.
“FDA has recognized that gas sterilization of duodenoscopes provides a greater margin of safety than high level disinfection,” said Andersen Products President and CEO, Ted May. “This is the exciting next generation technology – compact, high efficiency, rapid and low emission – offering healthcare facilities the proven safety and reliability of low-temperature gas sterilization. Hospitals have been looking for a safe, compatible and reliable process to sterilize packaged duodenoscopes, which this clearance delivers.”
The EOGas 4 510(k) clearance follows two national awards Andersen received in 2020 for its emission-reducing technology:
- FDA’s “Innovation Challenge Winner” for developing “strategies or technologies to reduce emissions to as close to zero as possible from the EO sterilization process.”
- National Steering Committee of the Small Business Environmental Assistance Programs and Small Business Ombudsmen’s “2020 Small Business Environmental Stewardship Award” recognizing Andersen’s high-efficiency, low-emission sterilization process and commitment to environmental stewardship.
EOGas 4 FDA 510(k) Resources
EOGas 4 510(k) – K192978
FDA Letter
Indications for Use
Summary
Endo-SteriTest Rapid BI 510(k) – K202879
(Self-contained biological indicator – 4 hour incubation)
Endo-SteriTest BI 510(k) – K192980
(Self-contained biological indicator)
FDA Letter
Indications for Use
Summary