Why Ethylene Oxide (EO/EtO) Sterilization?
Why Use Ethylene Oxide Sterilization?
You should have an Andersen gas sterilizer right next to your autoclave.
Why EO gas? Because it and your autoclave are a perfect complement! The autoclave is fast. The ethylene oxide (EO or EtO) sterilizer is gentle and compatible with far more materials. Both are proven technologies with a long track record of operator safety.


Gentle and Compatible
Sterilizing small batches in an Andersen gas sterilizer protects your most delicate instruments, electronics, drills, fiber optics, handpieces and cameras from any damage. As well as those plastic, fabric, cellulose or rubber items that you can’t sterilize with other methods. Most items—except food, drugs or liquids—can be sterilized. In fact, even your most delicate instruments can be sterilized without damage.
That’s right, zero damage. Low temperature, no steam, no deep vacuum, no oxidation, no dulling, no damage. All those ‘no’s’ add up to a huge positive! You’ve invested a lot in your equipment, keep it in like-new condition for many years to come.
EO can even sterilize through mated or threaded metal surfaces. It will permeate through sealed plastic, Tyvek® and other wrapping—leaving the objects inside sterilized and protected from contamination. After sterilization, your packs have a shelf life of up to 6 months!
Operator Safety
There are many ways our exclusive technology helps safeguard operator safety. But purely from the perspective of EO and operator safety, here are a few things to consider.
Sometimes it’s good to be “old.” Medical devices have been sterilized with EO since the 1950s. It’s a known quantity: Remarkably gentle and effective. That hasn’t changed in the last 70 years. In fact, last year, nearly half of all new medical devices were sterilized with EO.
With time comes knowledge and assurance. We understand the importance of monitoring for exposure to EO and how to do so effectively. Andersen encourages customers to monitor when they install a sterilizer, if they move or re-install it and once a year.
Our AN94 Vapor-Trak badges are manufactured to meet and exceed applicable OSHA and NIOSH accuracy requirements at both 8-hour TWA and 15-minute STEL levels. One badge is fastened to the employee’s lapel for 15 minutes and the other is hung in the immediate work area for 8 hours. Monitors are enclosed within a US postage-paid mailer for convenient return to an independent laboratory. Analysis of each monitor is performed promptly after the mailer is received (cost included). The laboratory analysis report is sent for your record keeping and an immediate email notification will accompany any elevated results.
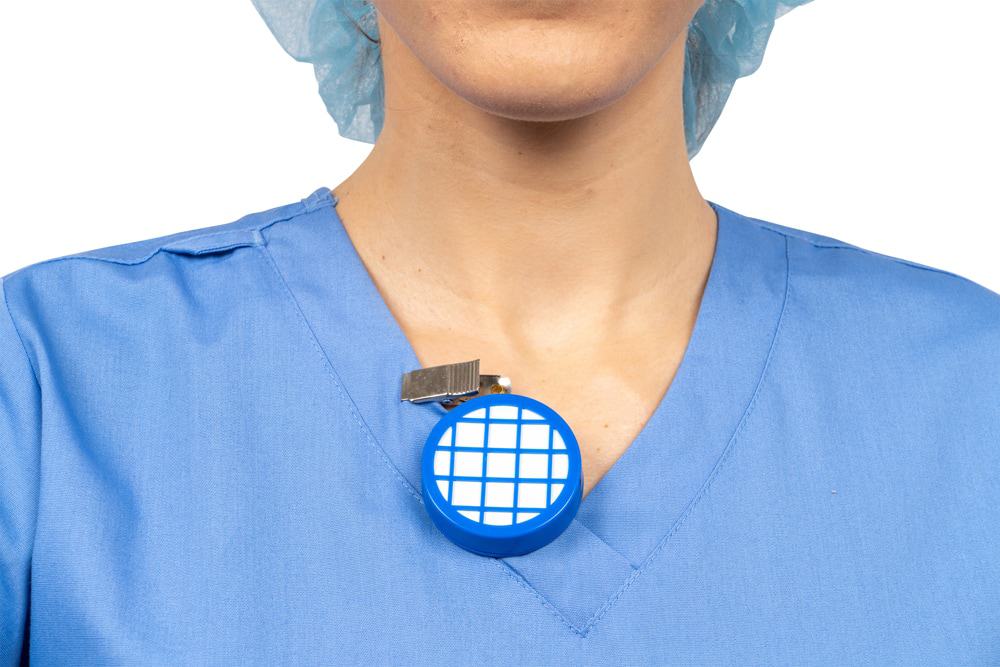
Elevated results are very uncommon but it does happen. It is occasionally an installation issue or a malfunction but more frequently it is a different chemical in the workplace.
Common chemicals that we take for granted are often as potentially dangerous as EO and can trigger the EO monitor. Say, bleach or – in a veterinary clinic – Parvosol, KennelSol or Rescue.
In one memorable instance, it ended up being an open bottle of glutaraldehyde!
Sterility Assurance Level
Let’s talk about sterility assurance level (SAL). It’s super technical, but you can handle it. In this industry, we can talk only about probability. You can’t ever say something is absolutely sterile because testing sterility includes a high chance of contamination.
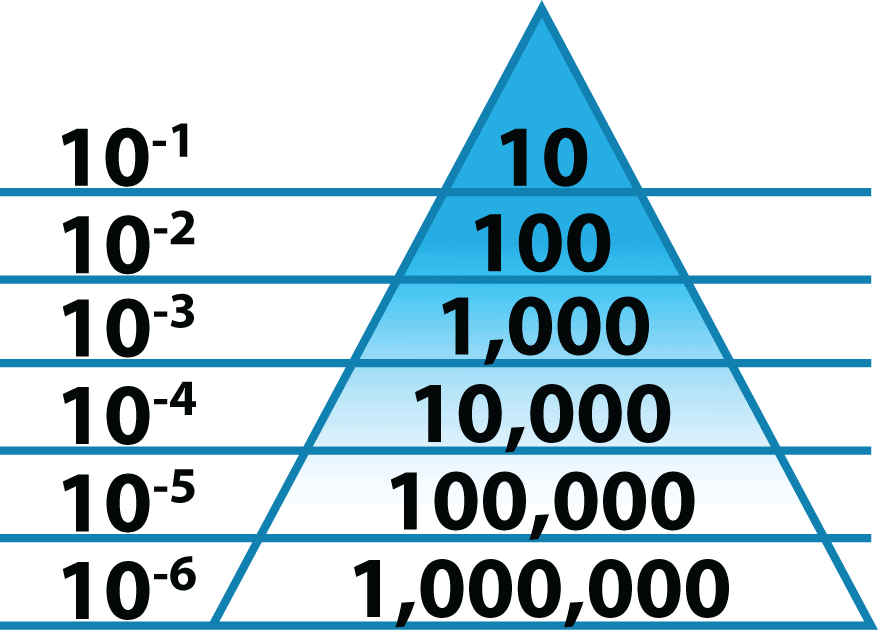
SAL addresses the likelihood of survival of a viable microorganism – such as bacteria, viruses or spores. Low-level sterilization receives a 10-3 SAL, high-level sterilization for heat-resistant medical instruments gets 10-4, whereas the standard for anything that enters the body anywhere other than through the digestive tract is 10-6. Each number is a magnitude greater. 10-3 SAL has a 1 in 1,000 chance a microorganism survived. 10-6 has a 1 in 1,000,000 chance.
Typically, articles on this topic delve into legal and regulatory implications and if the object is going inside someone’s body. But at Andersen, we think everyone deserves 10-6 SAL…including pets and other animals.
It doesn’t matter where it’s going. If you wanted an object disinfected, then you might as well actually achieve 10-6 terminal sterility. Our sterilizers are at a price point where a clinician can afford to use a sterile instrument for every patient.
Sterilant Toxicity Myths
In the last 10 years, hydrogen peroxide (H2O2) and peracetic acid (PAA) salespeople have spread a lot of misinformation about their sterilants and about ours – ethylene oxide (EO or EtO). In fact, we’ve done a whole separate article on H2O2‘s Big Lies.
H2O2 and PAA Myths:
- More environmentally friendly
- You have to buy their product because EO is going to be banned
- Not dangerous / less dangerous
- No monitoring required
Environmentally Friendly Myth
As far as “environmentally friendly,” the argument seems to be that H2O2 and PAA break down into harmless components that people don’t find scary. The implication seems to be if they end up breaking down into something harmless, they must be less scary overall. Not true, as it turns out (see below).
EO also breaks down into something harmless. Ethylene oxide is NOT classified as a greenhouse gas. When it is released into the atmosphere it dissipates quickly. EPA says, “Ethylene oxide reacts in the air to form formic acid, which is a naturally occurring chemical.” And, as Nature.com asserts, “Formic Acid is one of the simplest and most abundant organic molecules in Earth’s atmosphere…”
EO is Going to be Banned Myth
EO is NOT going to be banned. It is not banned in any state currently, nor are there any serious discussions to that end at any level. More than HALF of all new medical devices are sterilized with EO and many can only be sterilized via this method.
These rumors are more than 20 years old and stem from technology that is no longer in use (and Andersen never used). Some companies mixed CFCs and HCFCs with EO as propellants – they were banned from use in 1996. Talk about a tired rumor!
Please Note: Our goal is not to malign these sterilization methods. They have their undeniable strengths and may very well have their place in a well run SPD – if there is monitoring in place to protect staff. We just want to make sure we’re playing on a level field.
Leveling the Field
| Ethylene Oxide | Hydrogen Peroxide | Peracetic Acid | Glutaraldehyde | |
|---|---|---|---|---|
| OSHA 8hr/15min PEL: Permissible Exposure Limits | 1 ppm / 5 ppm | 1 ppm / – | – / – | – / – |
| ACGIH 8hr/15min TLV: Threshold Limit Values | 1 ppm / – | 1 ppm / – | – / 0.4 ppm | 0.05 ppm / – |
| NIOSH 8hr/10min REL: Recommended Exposure Limit | <0.1 ppm / 5 ppm | 1 ppm / – | – / – | 0.2 ppm / – |
| NIOSH IDLH: Immediately Dangerous to Life or Health | 800 ppm | 75 ppm | 0.55 ppm (proposed) | – / – |
| HSE 8hr/15min WEL: Workplace Exposure Limits | 1 ppm / – | 1 ppm / 2 ppm | – / – | 0.05 ppm / 0.05 ppm |
| Cancer Status | IARC: Carcinogenic to Humans ACGIH: Suspected Human Carcinogen | IARC: Not Classifiable as to Carcinogenicity in Humans ACGIH: Confirmed Animal Carcinogen with Unknown Relevance to Humans | ACGIH: Not Classifiable as a Human Carcinogen | – / – |
| Odor Threshold | 400 to 700 ppm | Almost no odor | 50 ppm | 0.04 ppm |
| CAMEO Datasheet | Ethylene Oxide | Hydrogen Peroxide | Peracetic Acid | Glutaraldehyde |
| Downsides | Cycle times (Andersen: 3.5 – 26 hours including standard aeration) | Acquisition, labor, operating and service costs, reliability issues, struggles to sterilize long lumens, pulls a deep vacuum that can damage delicate items, corrosive | Immersible instruments only, makes plastic brittle, corrodes metal, can only process one scope or a small number of instruments per cycle | |
| NOT compatible with | Food, drugs or liquids | Cloth, liquids, paper, powders, anodized aluminum and anything containing cellulose | Aluminium, carbon steel, some cross-linked polyethylene and metal alloys containing copper |
Not Dangerous Myth
As you can see, EO and H2O2 have the same Permissible Exposure Limit (PEL) of 1 ppm and the proposed PEL for PAA is even lower.
Regarding Immediately Dangerous to Life or Health (IDLH), the level for EO (800 ppm) is ten times higher than for H2O2 (75 ppm) – remember, a higher number represents a lower risk. And for PAA, most people are surprised to learn that NIOSH is considering a proposed IDLH value that is lower still.
EO is rated as having comparable or lower risk than these other common sterilants/disinfectants. When customers start considering EO’s enhanced compatibility, 10-6 SAL terminal sterility and the ability to easily test for cycle success and operator exposure — we start having a whole new conversation!
Keep in mind that PAA is a combination of H2O2 and Acetic Acid – both of which have limits set from all agencies above. Because of this ChemDAQ says, “It’s only a matter of time before OSHA, NIOSH and others reevaluate the PEL for Peracetic Acid to better protect workers from this hazardous chemical.”
No Monitoring Required Myth
H2O2 and PAA systems have now been around for over thirty years, and they have become the dominant sterilization and disinfection methods in most healthcare facilities. Organizations governing worker safety have begun enforcing monitoring requirements for both H2O2 and PAA (Under the OSHA General Duty Clause, employers have an obligation to protect workers from serious and recognized workplace hazards even where there is no standard.)
Everyone who operates an EO sterilizer understands the importance of monitoring and testing. Are you testing for these other potentially hazardous chemicals in the workplace?
Further in the Weeds
Here are a few more fun sterilant gas myths from ChemDAQ if you’re hungry for more! And, again, don’t miss the far more through H2O2‘s Big Lies & Little Chambers article – which not only debunks the most common lies but also takes a deep dive into feature comparison.
Vs. liquid chemical sterilization
Ethylene oxide (EO) and liquid chemical sterilization (LCS) are typically used for devices that would be sensitive to high heat used in steam sterilization, as well as rubber and plastic devices that can be damaged by irradiation.
Does that mean EO and LCS are just as effective?
LCS: FDA describes LCS as a 2-part process:
- Immerse the device in a liquid chemical germicide.
- Processed devices are rinsed with water to remove the chemical residues.
LCS has several limitations, as displayed in the table below.
EO sterilization: In contrast, EO achieves “traditional sterilization,” which FDA defines as a “validated process used to render a product free of all forms of viable microorganisms.” Further, FDA describes EO gas sterilization as “an important sterilization method that manufacturers widely use to keep medical devices safe.” It also points out, for many medical devices, “sterilization with (EO) may be the only method that effectively sterilizes and does not damage the device during the sterilization process.”
| Characteristics | Ethylene Oxide Gas Sterilization | Liquid Chemical Sterilization |
| A “traditional” sterilization process | ||
| SAL: 10-6 |  | |
| Process monitored using a BI |  | |
| Sterilized device is dry |  | |
| Device is wrapped, packaged providing a sterile shelf life |  | |
| Survival kinetics are well characterized |  |
LCS sterility assurance level: FDA states, that although “the rinse water is treated to minimize any bioburden, it is not sterile” and therefore devices rinsed with this water cannot be assumed to be sterile. “Furthermore, devices cannot be wrapped or adequately contained during processing in a liquid chemical sterilant,” rendering the devices prone to re-contamination.
EO Sterility assurance level: EO gas sterilization processes are designed by manufacturers with an associated sterility assurance level (SAL) of 10-6 — a level, according to the CDC, liquid chemical sterilants may not convey.
Biological Indicator use with LCS vs. EO: Another salient limitation, FDA states that biological indicators (BIs) are “not appropriate” for monitoring a LCS process (table above). FDA notes further that BIs “are generally used for monitoring traditional sterilization processes, like EO sterilization, where a SAL 10-6 is achieved. FDA has not cleared any (Bis) for monitoring (LCS) process.”
The survival kinetics for low-temperature sterilization methods (gas, vapor or plasma) and for thermal sterilization methods (steam and dry heat) have been studied and well characterized. Federal agencies acknowledge the kinetics for sterilization with liquid sterilants are less well understood. According to the CDC, “the survival curves for liquid chemical sterilants may not exhibit log-linear kinetics and the shape of the survivor curve may vary depending of the formulation, chemical nature and stability of the liquid chemical sterilant.”
A comparative study: Rutala et al. (1998) compared the sporicidal activity of four different low-temperature instrument processing technologies including two using EO with hydrochloro-fluorocarbons and a liquid chemical sterilant, respectively. These researchers reported that the former was “highly effective” in killing approximately 106 resistant Bacillus stearothermophilus spores present in the center of narrow-lumen stainless steel tubes.” This study found, however, that the liquid chemical sterilant process “was not effective in completely eliminating the 106 inoculum under test conditions.”
Unlike LCS, EO sterilization is a traditional sterilization technology that is associated with a SAL of 10-6, can be routinely monitored biologically, yields a dry, wrapped processed instrument that can be stored sterile with a shelf life – with an Andersen Sterilizer, FDA-cleared up to 6 months.
Soapbox
According to the FDA, liquid chemical sterilization is not associated with a SAL of any value even though a SAL value in the context of instrument sterilization is essential to know and define. The effectiveness of any process for sterilization may be questioned if it lacks a defined and published SAL. EO gas sterilization is associated with a SAL of 10-6 and is a process that can be monitored biologically and can be expected to consistently and reliably sterilize a wrapped instrument for invasive procedures.
Liquid chemical sterilization cannot be monitored biologically and therefore the process has no means of assuring effectiveness. It is not associated with a defined SAL and yields an instrument that the liquid process’ parameters do not require to be sterile. The process cannot reliably and consistently assure that the instrument has been successfully sterilized, according to the FDA, and this is at odds with the FDA’s own definition of sterilization.
Eo History timeline
- 1930s
EO insecticidal use gaining popularity. (Source)
- 1940s
- 1950s
Dr. Charles Rush Phillips research spearheads EO’s use to sterilize delicate instruments. The McDonald process was patented for medical devices. (Source)
- 1960s
Late in the decade, EO became the dominant chemical sterilant in major healthcare facilities.
- 1980s
OSHA sets EO exposure limits. (Source)
- 1990s
EO/CFC ban rumors. (Source)
- 2000s
- 2008
Outbreak at 2 hospitals in Highland County, Florida – As many as 70 exposed, 22 dead; legitimate issues with reprocessing. (Source)
- 2012
Additional outbreaks with hundreds infected and dozens killed.
- 2015
- LA Times runs expose uncovering CRE outbreak at UCLA – 8 infected, 3 dead, 179 patients exposed. (Source)
- FDA releases safety warnings about the use of duodenoscopes. (Source)
- FDA convenes panel of experts to consult on whether current reprocessing standards are adequate. Their conclusion is no. (Source)
- FDA recommends the use of EO for duodenoscope reprocessing. (Source)
- World’s first FDA registration for flexible chamber EO sterilizer. (Source)
- 2017
Renewed cleaning efforts for scopes not enough, triggering the search of most efficient methods by leading hospitals. (Source)
- 2020
FDA notifies facilities again that gas sterilization has a greater safety margin than HLD.
November: Andersen receives first and ONLY FDA clearance for terminal sterilization of duodenoscopes and colonoscopes. (Source)
- 2021
April: Urological endoscopes
June: Bronchoscopes
join the list of complex devices that cannot be dependably disinfected. FDA continues to remind facilities that gas sterilization has a greater safety margin than HLD.
Complex device reprocessing, superbugs and EO: A short history
Complex Devices that cannot be dependably disinfected:
Duodenoscopes are a type of upper gastrointestinal endoscope used during endoscopic retrograde cholangiopancreatography, or ERCP, to diagnose and treat disorders of the bile and pancreatic ducts.
Urological endoscopes are used for viewing and accessing the urinary tract.
Bronchoscopes may be used to examine, diagnose and treat disorders and diseases of the throat, larynx, trachea, and lower airways.
Which device will be next?
“Superbugs” are strains of bacteria, viruses, parasites and fungi that are resistant to most antibiotics and other medications commonly used to treat the infections they cause. These classes of antibiotics can include carbapenems – which are “last resort” antibiotics used to treat many types of serious infections caused by multidrug-resistant bacteria.1
Carbapenem-resistant Enterobacteriaceae – or CRE – are a particularly pernicious family of gram-negative bacteria resistant to these antibiotics. CRE and related superbugs have become a global public health scourge. The mortality rates for patients infected with CRE and certain other superbugs, particularly bloodstream infections, can be as high as 50% – or higher, in some patient subgroups.2,1
In February 2015, the FDA acknowledged for the first time, that duodenoscopes could remain persistently contaminated with life-threatening superbugs and related multidrug-resistant organisms.3 Examples of these potentially deadly microorganisms include CRE and carbapenem-resistant Pseudomonas aeruginosa.
During its subsequent review of several duodenoscope-related outbreaks, the FDA found that many of the superbug infections occurred despite staffers cleaning and disinfecting the duodenoscope in accordance with the manufacturer’s instructions.
According to the FDA, the complex physical design of duodenoscopes “may impede effective reprocessing,” ominously stressing that “meticulously cleaning duodenoscopes prior to high-level disinfection should reduce the risk of transmitting infection but may not entirely eliminate it.”3
This was a watershed finding because, previously, virtually every infection associated with a contaminated duodenoscope, or other type of flexible endoscope, had been attributed to a reprocessing breach – more specifically, to failure to clean and disinfect the device as instructed in its labeling. Reprocessing a flexible endoscope as prescribed by the manufacturer had always prevented cross-infection (with few exceptions), until that time first recognized in 2015.3
To combat the infection risk, enhance reprocessing and improve the safety of duodenoscopes, FDA advised healthcare facilities, in a safety communication published a few months later, in August 2015, to consider implementing one or more of four enhanced, or “supplemental,” measures, which included ethylene oxide sterilization.4
Drawing a distinction between disinfection and sterilization, FDA stated in this August communication “duodenoscopes should be subjected to high-level disinfection following manual cleaning after each use. When possible and practical, duodenoscopes should be sterilized due to the greater margin of safety provided by sterilization.”4
Indeed, subsequent field surveillance later confirmed ethylene oxide (EO) sterilization was the most effective of the supplemental measures, and validation studies showed that it was the only measure that assures the complete inactivation of highly resistant microorganisms.5
A continuing infection risk?
Some data suggest that the incidence of a duodenoscope transmitting microorganisms, including CRE and other potentially deadly superbugs, during ERCP decreased, at least for a time, following the FDA’s alerts in 2015 informing healthcare facilities and device manufactures about this public health risk.6
However, reports submitted to the FDA’s adverse event database for medical devices, or “MAUDE,” since 2015 indicate that, the number of FDA reports linking a duodenoscope to an infection apparently decreased in 2016 and even more so in 2017 compared to the relatively high number of cases reported to the FDA in 2015.6 But 2018 reversed the trend and in 2019 the FDA received twice as many reports.
(Deadly) Alphabet Soup
MDROs: Multidrug-resistant microorganisms. Strains of bacteria, viruses, parasites and fungi that are resistant to most antibiotics and other medications commonly used to treat the infections they cause. Also known as superbugs.
CRE: Carbapenem-resistant Enterobacteriaceae, a particularly pernicious superbug.
HAI: Hospital-acquired infection or nosocomial infection. Most superbugs are acquired in a healthcare setting.
Common Superbugs
Ratcheting up the stakes, a report published in 2019 linked a duodenoscope to the possible transmission of a superbug carrying the mcr-1 gene, which can confer colistin antibiotic resistance to the microorganism.7 Like carbapenems, colistin may be used as a “last line of defense” for treating some types of multidrug-resistant infections. Notably, some colistin-resistant infections may be untreatable.
Several factors may explain these troubling trends, but one clear point warrants particular attention: the risk of infection associated with use of a duodenoscope remains a concern today.
Indeed, according to the CDC, more healthcare-associated outbreaks have been linked to flexible endoscopes than to any other type of medical device.
CDC
FDA published alerts in 2019 and 2020 recommending that healthcare facilities and manufacturers “begin transitioning to duodenoscopes with disposable components” and, in the later alert, stressed “sterilization, particularly terminal (e.g., gas) sterilization, provides a greater margin of safety than high-level disinfection.”9
Andersen solved the problem
We hate to toot our own horn but sometimes you need to just say it. In 2015, Andersen Sterilizers had a duodenoscope clearance underway with the FDA. Because of the CRE outbreak, we were forced to withdraw our claim. At the time the agency wasn’t sure of source of infections or how to test these complicated instruments post-sterilization.
We spent the next two years negotiating testing protocols with the FDA. During this time duodenoscopes were redefined as a “collection of instruments.” A new validation and simulated use testing protocol was chosen: The duodenoscope is inoculated at 7 of the hardest to reach sites in the scope with 106 bacterial spores.
This is a significantly higher bar than any previous FDA requirement. We are very proud to have achieved it.
Andersen validated duodenoscopes from all three major manufacturers and a colonoscope with an 11’6″ channel x 1.2mm!
Bronchoscopes and urological endoscopes pose a risk, too
Early in 2021, FDA acknowledged that other types of flexible endoscopes also remain a concern and may pose a risk of infecting patients with superbugs and related multidrug-resistant organisms.
In April, FDA issued a letter to raise awareness among healthcare providers “about the risk of infections associated with reprocessed urological endoscopes, including cystoscopes, ureteroscopes, and cystourethroscopes.”10
FDA wrote this April letter after acknowledging receipt of “over 450” medical device reports describing “patient infections post procedure or other possible contamination issues associated with reprocessing these devices.”10
Similar to the circumstances surrounding the infections linked to “reprocessed” duodenoscopes, FDA concluded in the letter that both the reprocessing instructions and designs of urological endoscopes could be factors contributing to these reported cases of infection and device contamination. Similar to earlier alerts, the FDA advises facilities to follow IFUs, maintain their devices correctly and gives two reprocessing choices – one of which includes sterilization.10
Two months later in June, FDA published a safety communication alerting the public to the risk of a third type of flexible endoscope ― reprocessed bronchoscopes ― similarly infecting patients with multidrug-resistant bacteria, again including CRE.11 The FDA’s notice provides updated advice that supplements the FDA’s earlier (and now outdated) alert, published in September 2015, also discussing infections linked to reprocessed (flexible) bronchoscopes.12
This June alert focusing on bronchoscopes provided two new recommendations that were not discussed in the earlier 2015 communication: first, that healthcare facilities “consider using sterilization instead of high-level disinfection when feasible because sterilization has a greater safety margin than high-level disinfection.” Otherwise, if “sterilization is not available,” then high-level disinfection of bronchoscopes should be performed.11
Second, the FDA’s June alert recommends that healthcare facilities “not use damaged devices or those that have failed a leak test, as they could be a potential source of contamination.” Examples of endoscope damage include loose parts; damaged channel walls; and other signs of wear or damage.11
Soapbox
The June FDA alert also says, “Consider using a single-use bronchoscope in situations where there is an increased risk of spreading infection (for example, multidrug-resistant microorganisms, immunocompromised patients, or patients with prion disease) or when there is no support for immediate reprocessing of the bronchoscope.”
The FDA is advising the use of a type of endoscope (reusable, single-use) based on the immuno-status of the patient. This can introduce two standards of care, which is problematic. Sterilizing with EO resolves this issue.
Moreover, is the FDA stating that in situations where there is no perceived increased risk of spreading infection, that single-use bronchoscopes not necessarily be considered? This is confusing … Who is to decide whether there is an increased risk, and what defines “increased,” other than the broad definition in the quote? What about undetected infections?
Growing support
The American Society for Gastrointestinal Endoscopy updated their “Multisociety guideline on reprocessing flexible GI endoscopes and accessories” in 2021 to state, “The use of EtO sterilization on duodenoscopes during infectious outbreaks has been associated with terminating these outbreaks and such a modality should be considered in selected settings and patient populations.”14
The Multisociety guideline goes on to reference the work of Larry Muscarella, a frequent collaborator here at Andersen, “EtO sterilization of duodenoscopes can be an effective tool in some clinical situations, specifically when there are infectious outbreaks observed among patients who have undergone ERCP. A systematic review by Muscarella examined all reported carbapenem-resistant Enterobacteriaceae–and multidrug-resistant organisms–related infections in the United States and Europe attributed to duodenoscope exposure and assessed the adequacy of reprocessing in these outbreaks.
Factors such as endoscope design, lapses in reprocessing guidelines, damage to the endoscope, or a lack of servicing, maintenance, and repair of the endoscope were hypothesized to be contributors to these outbreaks. In this review, 6 of 17 studies implemented EtO sterilization as an intervention during infectious outbreaks; in at least 3 and possibly 6 studies this intervention yielded an absence of culture positivity from duodenoscopes at all sites, thereby stopping the outbreaks. Similar data exist for EtO sterilization ceasing outbreaks attributed to bronchoscopes. Therefore, the implementation of EtO sterilization appears to be an effective tool for terminating carbapenem-resistant Enterobacteriaceae and multidrug-resistant organism outbreaks associated with duodenoscopes.”
Soapbox
The Multisociety guideline goes on to say, “However, sterilization technology is costly, inefficient, and associated with potential toxicity to reprocessing personnel and surrounding communities; additionally, there are concerns about endoscope performance and durability, and such technology is not widely available.”
In its 4 Supplemental Measures document (2015) FDA says, “Implementing EtO gas sterilization is costly and the process may not be readily available in or accessible to all health care facilities. EtO may affect the material and mechanical properties of the duodenoscope.”
It seems both companies are describing processes from a bygone era – the old 50 lb tank systems of old, perhaps. In any case, they describe precisely what an Andersen sterilizer is not. Our sterilizers are affordable, and incredibly efficient due to our exclusive technology, present a very comparable risk to operators and the surrounding community to other sterilization/disinfection methods, are so incredibly gentle we say the process is “zero damage” and, of course, they’re readily available.
Conclusions
The risk of superbug infections associated with reprocessed duodenoscopes, bronchoscopes and urological endoscopes, among other types of flexible endoscopes, remains a concern today, warranting attention and review of the FDA’s recently published alerts, along with manufacturer instructions, to mitigate this risk.
Failure to properly reprocess a flexible endoscope has been directly linked to deadly infections of CRE, carbapenem-resistant P. aeruginosa and related superbugs.13 The mortality rates of superbug infections can be as high as 50%, or higher.11 Cleaning and disinfecting the endoscope correctly, in accordance with manufacturer instructions, significantly reduces the infection risk, but may not entirely eliminate it, according to the FDA. 3,12
The FDA has repeatedly (most recently in June) recommended ethylene oxide gas sterilization over high-level disinfection for reprocessing these endoscopes because of its “greater safety margin.”11 Numerous studies have documented that EO sterilization of endoscopes during infectious outbreaks has been associated with terminating these outbreaks.14
Andersen holds the ONLY FDA clearance for terminal sterilization for duodenoscopes and colonoscopes, with a maximum lumen length of 3530 mm (11.6 feet) and minimum lumen diameter of 1.2 mm (the thickness of a Blu-Ray, less than the height of a dime).
We also hold FDA-clearances for urological endoscopes and bronchoscopes, including: bronchovideoscopes, cystoscopes, ureteroscopes, choledocoscopes, gastrovideoscopes and gastrointestinal videoscopes.
recap
- EO is the FDA-recommended method for reprocessing flexible endoscopes because of its greater margin of safety
- Andersen holds recent (post-2015) FDA clearances for flexible endoscopes in all of troublesome categories. In some cases we hold the only clearance.
- We hold clearances for chemical and biological indicators and packaging that maintain the sterility of endoscopes for 3-6 months after reprocessing.
- Our sterilizers are tabletop (two larger models available). They are easy to install and use – with free training for the life of your sterilizer. An Andersen Sterilizer is a seamless addition to your practice.
Is it time to protect your patients and safeguard your business?
Vs. other eo options
Wait! Why Andersen? We thought you might ask that. If you’re looking at other companies that offer EO, they fall into one of two categories – they are a BIG household name or they’re a fly-by-night company based overseas.
| Andersen EOGas 4 | BIG Name Company | Fly-by-Night Overseas Company | |
| FDA Clearance | | | |
| Cabinet size | 29.5″ L x 22″W x 28″ H | 38″ L x 20″ W x 18″ H | 30.1″ L x 17.7″ W x 24.8″ H |
| Chamber size | 104 L | 224 L | 92 L |
| No external air compressor needed (less expensive installation) | | | |
| Abatement method | Cationic | Catalytic | None Offered |
Regarding abatement, another option uses a catalytic method – which is an exothermic (heat-producing) reaction to convert EO exhaust into CO2 and water vapor. This process “burns off” the EO. They are expensive and complicated to install. Since there is flame involved and EO is highly flammable, there is always the small chance that a mistake will allow flame back into the chamber, causing it to explode. In contrast, our abatement method uses no flame and is inexpensive to install.
Please notice if companies use the word “cleared” but do not say by whom. Neither of these processes are FDA-cleared.

